Modified Release Nutraceutical Formulations: The Role of Benecel™ HPMC
There is a growing need for modified release dosage forms in the ever-changing sector of nutraceuticals. By lowering dosage frequency and guaranteeing a consistent release of active components, modified release formulations improve patient compliance. This paper investigates the potential use of BenecelTM Hydroxypropylmethylcellulose (HPMC), a water-soluble, high-purity cellulose ether, in the production of efficient modified release nutraceuticals. Benecel HPMC conforms to the strict guidelines established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the EC Commission Directive, and the Food Chemicals Codex.
Properties of Benecel™ HPMC
Hypromellose, or Benecel HPMC, is a non-ionic polymer whose viscosity and hydration are not affected by the pH of the surrounding solution. Because of this feature, it is especially well-suited for nutraceutical compositions where dependability and consistency are crucial. The particular Benecel HPMC grades under discussion are K35M and K200M. In a 2% solution, they show nominal harmonized viscosity ranges of 26,250 – 49,000 mPa·s and 150,000 – 280,000 mPa·s, respectively.
Glucosamine/Chondroitin Sulfate Formulations
Glucosamine and chondroitin sulfate are well-known joint maintenance supplements that typically require multiple doses daily. By utilizing Benecel HPMC, we aim to create longer-acting formulations, thus reducing dosing frequency and improving patient adherence.
Formulation and Compression
Two main formulations with 15% and 20% HPMC content were created, each comprising 500 mg of glucosamine and 400 mg of chondroitin sulfate. The processes of wet granulation and direct compression were applied to these compositions. Blending glucosamine, chondroitin sulfate, and Benecel HPMC was the first step in the direct compression process. Stearic acid was then added, and the tablets were formed using an instrumented Manesty Beta press.
The powder mixture was wetted, dried, ground, and then compressed into tablets for the wet granulation process. Physical characteristics such as thickness, weight, and crushing force were evaluated in the resulting tablets from both procedures.
Release Profiles and Tablet Physicals
The USP Apparatus I (basket) was used to measure the glucosamine release profiles from the tablets at 100 rpm in a USP pH 1.5 buffer. The release rate was somewhat delayed when the HPMC content was increased from 15% to 20%, as seen in Figures 1 and 2. The viscosity of HPMC, however, had no appreciable impact on the release rates.
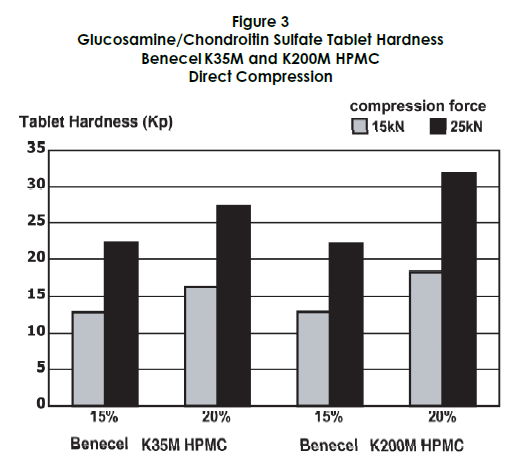
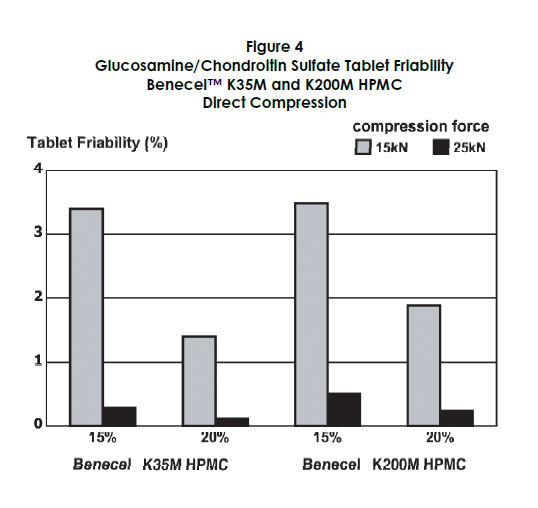
Tablet hardness and friability were also assessed. Higher levels of HPMC contributed to increased tablet hardness, while acceptable friability was maintained at higher compression forces, as shown in Figures 3 and 4.
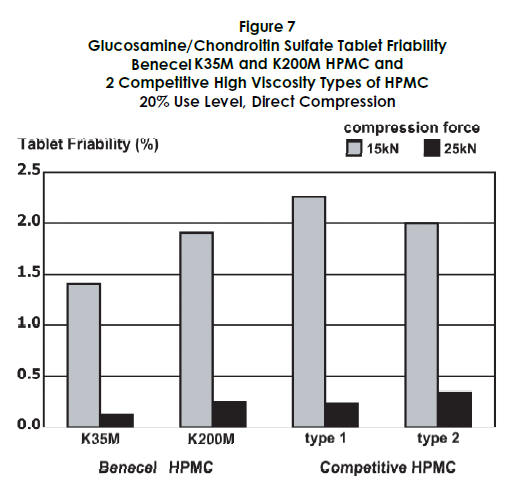
Competitive Comparison
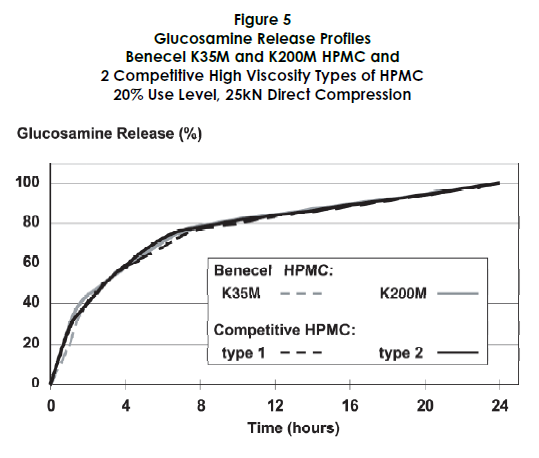
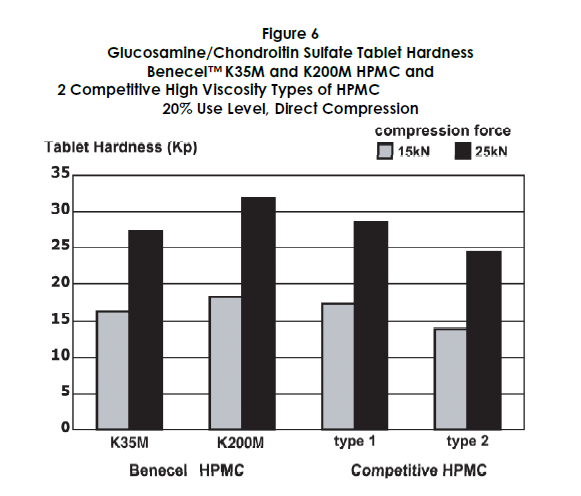
The performance of Benecel HPMC was benchmarked against two competitive high viscosity HPMC products. Figures 5 through 7 demonstrate that Benecel HPMC provides comparable or superior release profiles, tablet hardness, and friability.
Vitamin B6 Formulations
To further validate the efficacy of Benecel HPMC, a modified release formulation for vitamin B6, a highly soluble active, was developed. The formulation consisted of 75mg of vitamin B6 per 300mg tablet, with varying HPMC content (15%, 20%, and 30%).
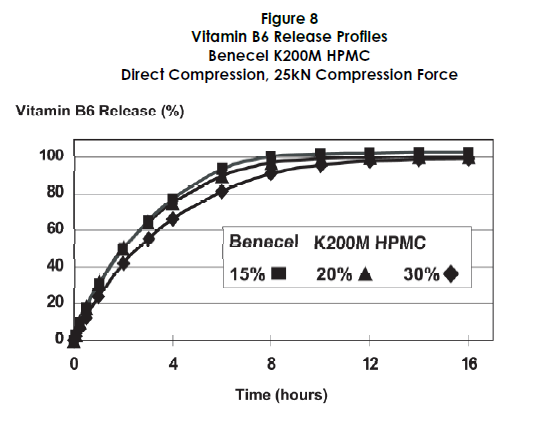
Formulation and Testing
The vitamin B6 formulations were directly compressed and tested for dissolution rates and physical properties. The dissolution studies showed that Benecel HPMC effectively retarded the release of vitamin B6, with 40-50% release at two hours and 66-78% at four hours (Figure 8).
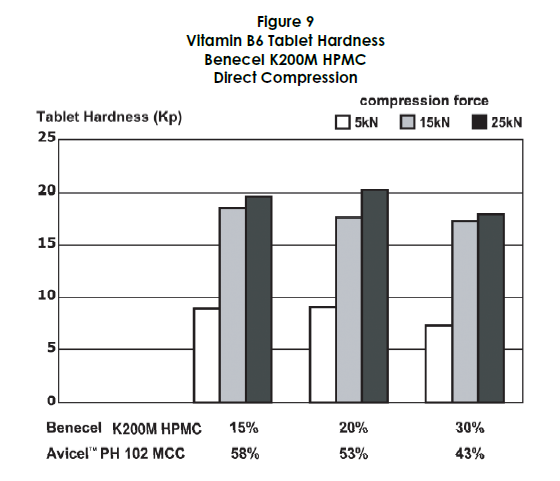
Tablet Physicals
The hardness of the vitamin B6 tablets remained consistent across different levels of HPMC, likely due to the high proportion of microcrystalline cellulose in the formulation. Friability was negligible for all formulations tested (Figure 9).
Benecel™ HPMC proves to be an excellent choice for modified release nutraceutical formulations. It effectively retards the release of highly soluble actives and ensures robust tablet physical properties. Both direct compression and low shear wet granulation methods yield consistent results, making Benecel HPMC a versatile and reliable component in the nutraceutical industry.

